Ocean Acidification
As the ocean absorbs carbon dioxide from the atmosphere, it becomes more acidic, threatening marine ecosystems and organisms that build shells and skeletons.
Key Statistics
pH Decrease Since Industrial Era
0.0 units
Since the onset of the industrial revolution, the ocean's surface pH has decreased by approximately 0.1 units.
Increase in Acidity
0%
The 0.1 unit decrease in pH represents a roughly 30% increase in acidity on the logarithmic pH scale.
CO₂ Absorption by Oceans
0%
The ocean absorbs about 30% of the carbon dioxide that is released in the atmosphere, leading to acidification.
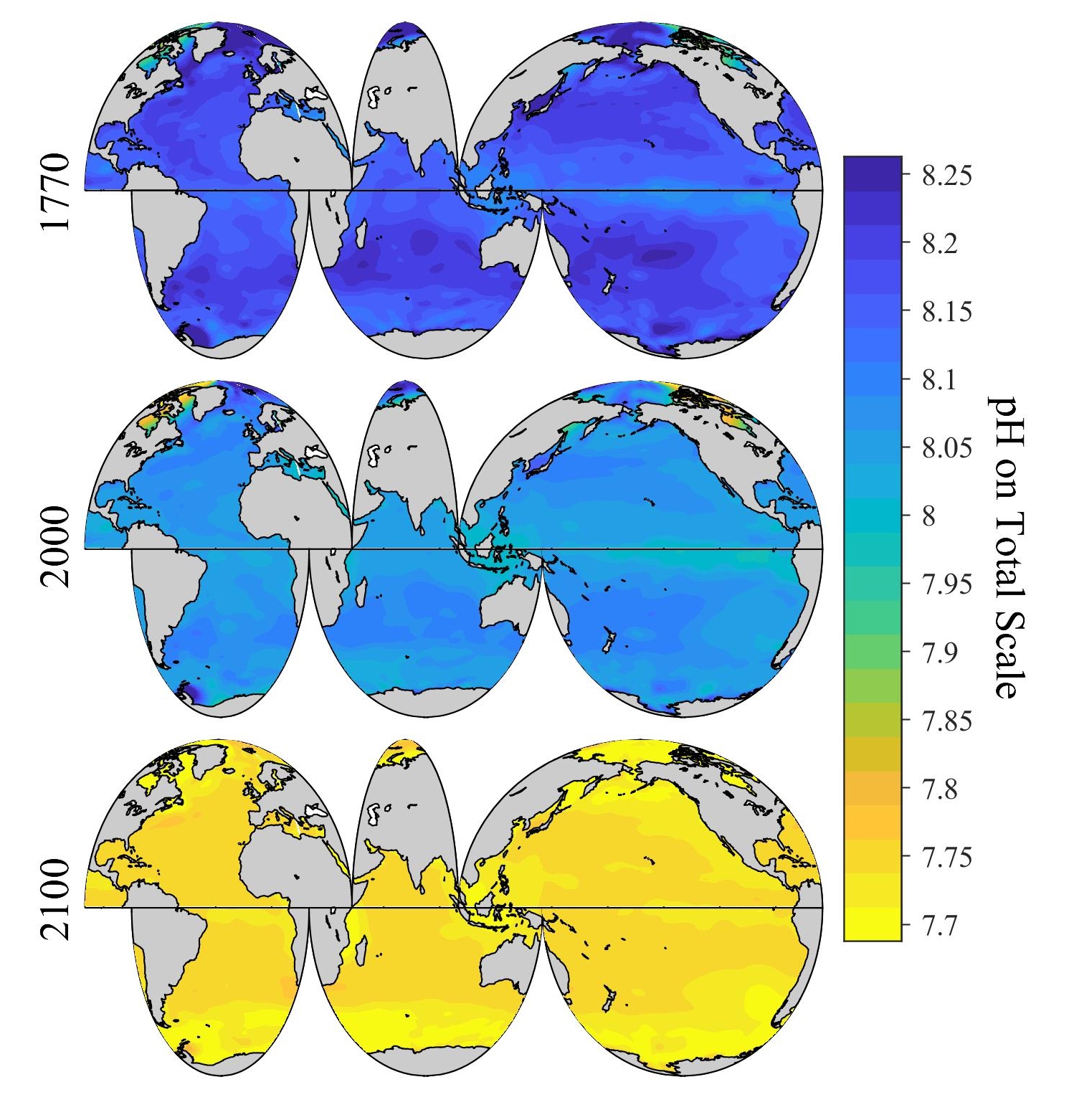
Ocean pH Trends
Data source: NOAA. The ocean's average pH is now around 8.1, which is basic (or alkaline), but as the ocean continues to absorb more CO₂, the pH decreases and the ocean becomes more acidic.
How Ocean Acidification Works
The Chemical Process
- CO₂ Absorption: Atmospheric carbon dioxide (CO₂) dissolves in seawater.
- Carbonic Acid Formation: CO₂ reacts with water to form carbonic acid (H₂CO₃).
- Acid Dissociation: Carbonic acid dissociates into hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻).
- Increased Acidity: The release of hydrogen ions increases the acidity of the water (lower pH).
- Carbonate Ion Reduction: Hydrogen ions bond with carbonate ions (CO₃²⁻), reducing their availability for marine organisms.
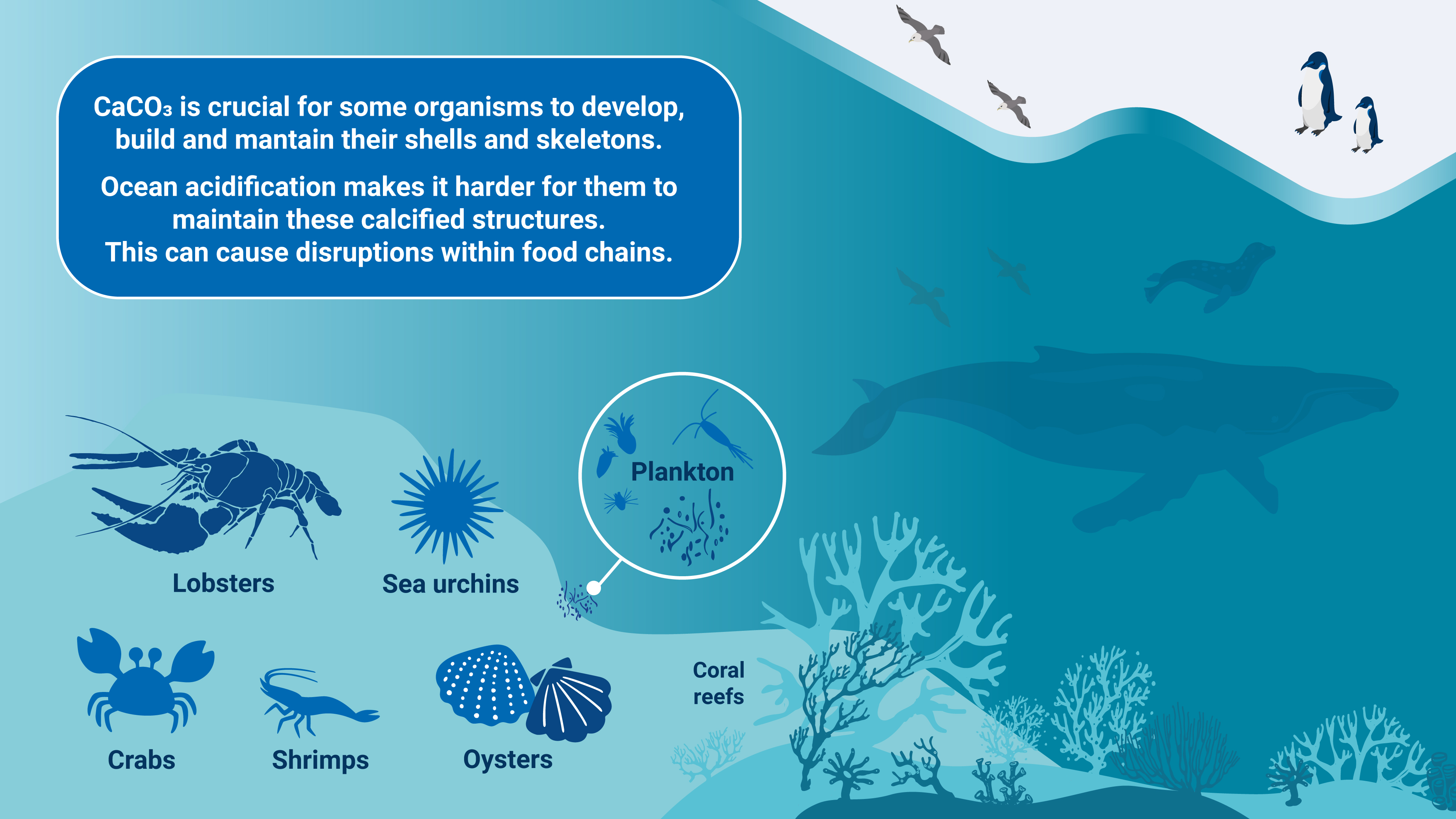
Impacts on Marine Life
Effects on Calcifying Organisms
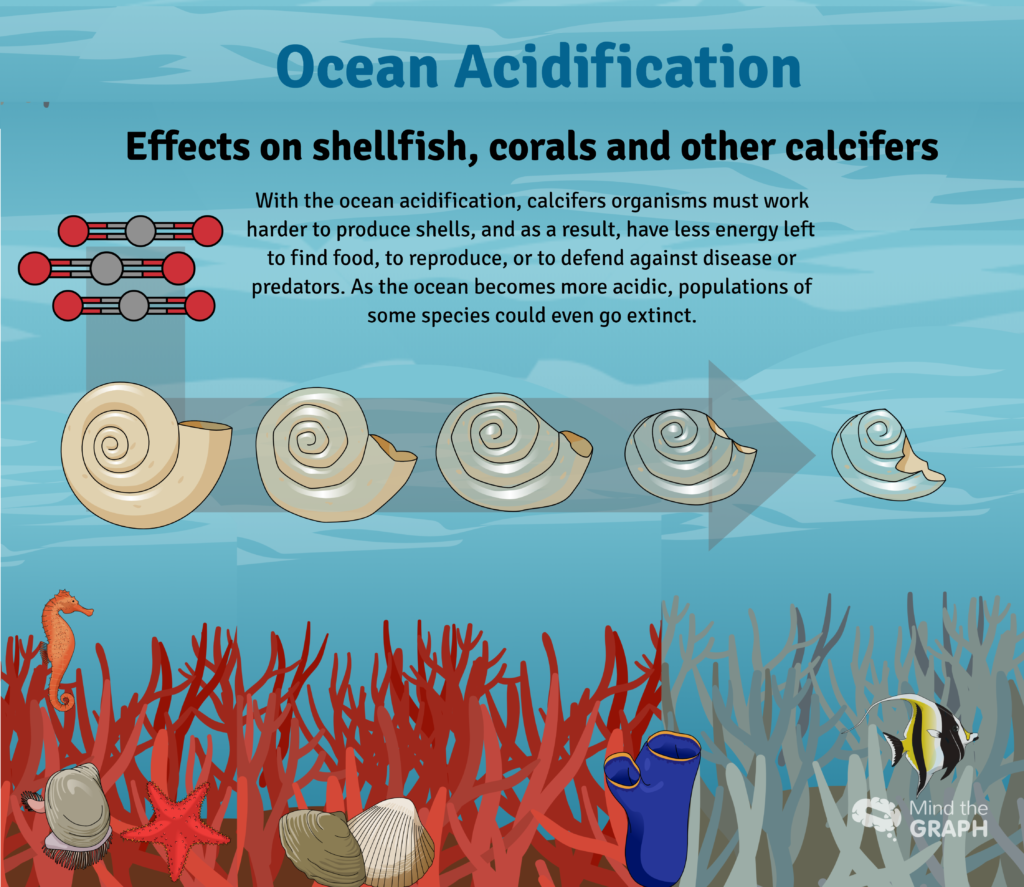
Ocean acidification is already impacting many ocean species, especially organisms like oysters and corals that make hard shells and skeletons by combining calcium and carbonate from seawater.
- • Reduced calcification rates in coral reefs, shellfish, and other marine organisms
- • Shell dissolution in pteropods (sea butterflies) and other sensitive species
- • Weakened structures in coral reefs, making them more vulnerable to storms and erosion
Broader Ecosystem Effects

Beyond direct impacts on shell formation, ocean acidification affects marine ecosystems in multiple ways:
- • Changes in fish behavior and sensory abilities, including reduced predator avoidance
- • Disrupted food webs as key species are affected
- • Potential shifts in species composition and ecosystem functioning
- • Compounding effects when combined with ocean warming and deoxygenation
Future Projections
Projected pH Changes
Estimates of future carbon dioxide levels, based on business-as-usual emission scenarios, indicate that by the end of this century the surface waters of the ocean could have a pH around 7.8, the lowest in 14-17 million years.
Regional Variations
Ocean acidification is not uniform across all ocean regions. Some areas are experiencing more rapid changes due to local conditions:
- Polar Regions: Cold water absorbs more CO₂, making Arctic and Antarctic waters particularly vulnerable to acidification.
- Upwelling Zones: Areas with natural upwelling of deep water (e.g., U.S. West Coast) already experience more acidic conditions.
- Coastal Areas: Runoff, pollution, and eutrophication can exacerbate acidification in coastal waters.
Monitoring and Research
Scientists employ multiple observational platforms for monitoring ocean acidification to better understand its progression and impacts.
Monitoring Methods
- • Buoys and Moorings: Recording surface CO₂ and pH every three hours across coastal, open-ocean, and coral reef environments.
- • Hydrographic Cruises: Long-term cruises documenting OA progression, revealing spatial and temporal variability.
- • Volunteer Observing Ships: Commercial vessels providing large-scale trend data on ocean chemistry.
- • Autonomous Systems: Moorings, Saildrones, and Argo floats collecting high-frequency data on ocean chemistry.
Research Focus Areas
- • Biological Responses: Studying how different species and ecosystems respond to changing ocean chemistry.
- • Adaptation Potential: Investigating whether marine organisms can adapt to more acidic conditions over time.
- • Ecosystem Services: Assessing impacts on fisheries, aquaculture, and coastal protection provided by reefs.
- • Mitigation Strategies: Exploring approaches to reduce CO₂ emissions and potentially restore ocean chemistry locally.
Economic Impacts
Ocean acidification threatens industries and communities that depend on healthy marine ecosystems, with significant economic implications.
Shellfish Aquaculture
The U.S. shellfish industry has already experienced significant losses due to acidification, particularly in the Pacific Northwest where oyster hatcheries have seen up to 80% reduction in production during acidification events.
Coral Reef Tourism
Coral reefs generate billions in tourism revenue globally. Degradation from acidification and warming threatens this income source for many coastal communities and small island nations.
Fisheries
Changes in marine food webs due to acidification could impact commercial fish stocks, affecting food security and livelihoods for millions of people who depend on fishing.